Contact Information
Location: GSRC 431
Phone: 803-777-8143
Email: wiskur@mailbox.sc.edu
ORCID
Research Highlights
Intermolecular Catalyst and Substrate Interactions
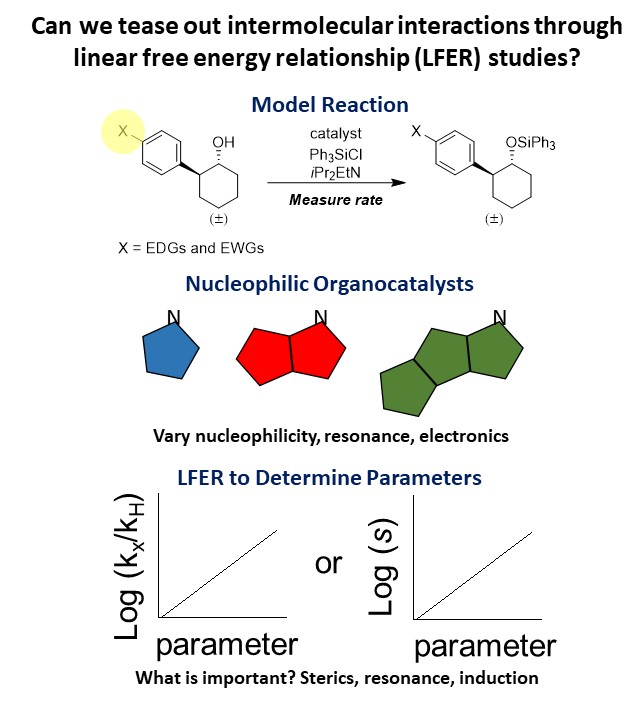
Organocatalysis has become an important part of asymmetric catalysis over the last
20 years, catalyzing a wide variety of transformations. Many of these catalysts are
selective due to intermolecular interactions between the catalyst and the substrate,
which preorganize the two to lead to selective products. Our research explores these
interactions, looking to understand the types of interactions present and how modifications
to the catalyst or substrate affect these interactions. We focus on nucleophilic imidazoles
and isothioureas to explore cation-pi interactions and we have discovered other surprising
interactions along the way. By using a model silylation reaction and linear free energy
relationships (LFERs) we can identify novel trends, which provides researchers with
a more comprehensive understanding of these catalysts to aid in future catalyst development.
Tuning Polymer Microenvironments
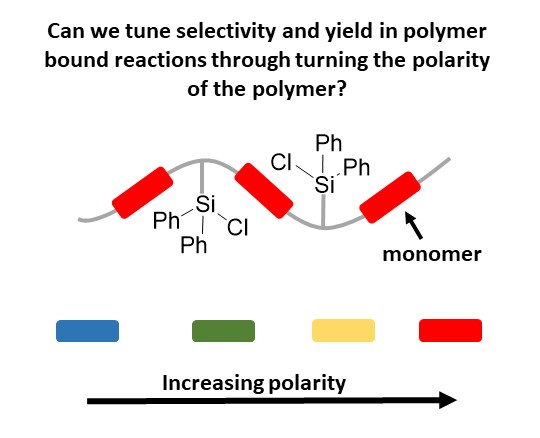
Small molecule catalysis plays a large role in organic synthesis, but one of the drawbacks
for solution-based catalysis is the purification of the products on large scale. If
companies need to run multi-gram reactions, being able to perform filtration to obtain
the product is more desirable than performing chromatography separation. One solution
to this problem is the development of polymer supported reagents or catalysts that
allow for their facile separation at the end of the reaction.
Unfortunately, not all small molecule catalysis translates well to polymer bound,
with frequent loss of selectivity and yield. One theory regarding why this occurs,
is the microenvironment of the polymers is different from the surrounding solution,
specifically polarity. Our work focuses on tuning that microenvironment, by altering
the polarity near reaction centers through incorporation of different monomers into
the polymer. The monomers bring the surrounding microenvironment closer to the desired
solvent polarity, such that the catalysis regains selectivity and yield.